Diffusion
Diffusion of innovation research was first started in 1903 by seminal researcher Gabriel Tarde, who first plotted the S-shaped diffusion curve. Tarde defined the innovation-decision process as a series of steps that includes:[37]
- First knowledge
- Forming an attitude
- A decision to adopt or reject
- Implementation and use
- Confirmation of the decision
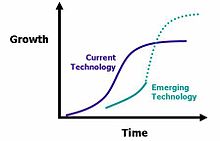
Once innovation occurs, innovations may be spread from the innovator to other individuals and groups. This process has been proposed that the life cycle of innovations can be described using the 's-curve' or diffusion curve. The s-curve maps growth of revenue or productivity against time. In the early stage of a particular innovation, growth is relatively slow as the new product establishes itself. At some point customers begin to demand and the product growth increases more rapidly. New incremental innovations or changes to the product allow growth to continue. Towards the end of its lifecycle, growth slows and may even begin to decline. In the later stages, no amount of new investment in that product will yield a normal rate of return
The s-curve derives from an assumption that new products are likely to have "product life" – i.e., a start-up phase, a rapid increase in revenue and eventual decline. In fact the great majority of innovations never get off the bottom of the curve, and never produce normal returns.
Innovative companies will typically be working on new innovations that will eventually replace older ones. Successive s-curves will come along to replace older ones and continue to drive growth upwards. In the figure above the first curve shows a current technology. The second shows an emerging technology that currently yields lower growth but will eventually overtake current technology and lead to even greater levels of growth. The length of life will depend on many factors.[38]
Measures
Edison et al.[8] in their review of literature on innovation management found 232 innovation metrics. They categorized these measures along five dimensions i.e. inputs to the innovation process, output from the innovation process, effect of the innovation output, measures to access the activities in an innovation process and availability of factors that facilitate such a process.[8]
There are two different types of measures for innovation: the organizational level and the political level.
Organizational level
The measure of innovation at the organizational level relates to individuals, team-level assessments, and private companies from the smallest to the largest company. Measure of innovation for organizations can be conducted by surveys, workshops, consultants, or internal benchmarking. There is today no established general way to measure organizational innovation. Corporate measurements are generally structured around balanced scorecards which cover several aspects of innovation such as business measures related to finances, innovation process efficiency, employees' contribution and motivation, as well benefits for customers. Measured values will vary widely between businesses, covering for example new product revenue, spending in R&D, time to market, customer and employee perception & satisfaction, number of patents, additional sales resulting from past innovations.[39]
Political level
For the political level, measures of innovation are more focused on a country or region competitive advantage through innovation. In this context, organizational capabilities can be evaluated through various evaluation frameworks, such as those of the European Foundation for Quality Management. The OECD Oslo Manual (1995) suggests standard guidelines on measuring technological product and process innovation. Some people consider the Oslo Manual complementary to the Frascati Manual from 1963. The new Oslo manual from 2005 takes a wider perspective to innovation, and includes marketing and organizational innovation. These standards are used for example in the European Community Innovation Surveys.[40]
Other ways of measuring innovation have traditionally been expenditure, for example, investment in R&D (Research and Development) as percentage of GNP (Gross National Product). Whether this is a good measurement of innovation has been widely discussed and the Oslo Manual has incorporated some of the critique against earlier methods of measuring. The traditional methods of measuring still inform many policy decisions. The EU Lisbon Strategy has set as a goal that their average expenditure on R&D should be 3% of GDP.[41]
Indicators
Many scholars claim that there is a great bias towards the "science and technology mode" (S&T-mode or STI-mode), while the "learning by doing, using and interacting mode" (DUI-mode) is ignored and measurements and research about it rarely done. For example, an institution may be high tech with the latest equipment, but lacks crucial doing, using and interacting tasks important for innovation.[citation needed]
A common industry view (unsupported by empirical evidence) is that comparative cost-effectiveness research is a form of price control which reduces returns to industry, and thus limits R&D expenditure, stifles future innovation and compromises new products access to markets.[42] Some academics claim cost-effectiveness research is a valuable value-based measure of innovation which accords "truly significant" therapeutic advances (i.e. providing "health gain") higher prices than free market mechanisms.[43] Such value-based pricing has been viewed as a means of indicating to industry the type of innovation that should be rewarded from the public purse.[44] Research and Development Expenditure Credit
An Australian academic developed the case that national comparative cost-effectiveness analysis systems should be viewed as measuring "health innovation" as an evidence-based policy concept for valuing innovation distinct from valuing through competitive markets, a method which requires strong anti-trust laws to be effective, on the basis that both methods of assessing pharmaceutical innovations are mentioned in annex 2C.1 of the Australia-United States Free Trade Agreement.[45][year needed][46][47]
Indices
Several indices attempt to measure innovation and rank entities based on these measures, such as:
- The Bloomberg Innovation Index
- The "Bogota Manual"[48] similar to the Oslo Manual, is focused on Latin America and the Caribbean countries.[citation needed]
- The "Creative Class" developed by Richard Florida[citation needed]
- The EIU Innovation Ranking
- The Global Competitiveness Report
- The Global Innovation Index (GII), by INSEAD[49]
- The Information Technology and Innovation Foundation (ITIF) Index
- The Innovation Capacity Index (ICI) published by a large number of international professors working in a collaborative fashion. The top scorers of ICI 2009–2010 were: 1. Sweden 82.2; 2. Finland 77.8; and 3. United States 77.5.[50]
- The Innovation Index, developed by the Indiana Business Research Center, to measure innovation capacity at the county or regional level in the United States.[51]
- The Innovation Union Scoreboard
- The innovationsindikator for Germany, developed by the Federation of German Industries (Bundesverband der Deutschen Industrie) in 2005[52]
- The INSEAD Innovation Efficacy Index[53]
- The International Innovation Index, produced jointly by The Boston Consulting Group, the National Association of Manufacturers and its nonpartisan research affiliate The Manufacturing Institute, is a worldwide index measuring the level of innovation in a country. NAM describes it as the "largest and most comprehensive global index of its kind".[citation needed]
- The Management Innovation Index - Model for Managing Intangibility of Organizational Creativity: Management Innovation Index[54]
- The NYCEDC Innovation Index, by the New York City Economic Development Corporation, tracks New York City’s "transformation into a center for high-tech innovation. It measures innovation in the City’s growing science and technology industries and is designed to capture the effect of innovation on the City’s economy."[55]
- The Oslo Manual is focused on North America, Europe, and other rich economies.
- The State Technology and Science Index, developed by the Milken Institute, is a U.S.-wide benchmark to measure the science and technology capabilities that furnish high paying jobs based around key components.[56]
- The World Competitiveness Scoreboard[57]